The BART Foundation aims to promote better outcomes for brain injury survivors by answering three questions: Which alternative therapies are likely to work, where can they be found, and how can they be afforded? One way we fulfill our mission is by carefully monitoring global research and clinical trial outcomes and sharing that information in user-friendly language with the TBI/ABI community.
While transcranial magnetic stimulation (TMS) is not currently listed as an alternative therapy that we officially support (it’s on our watch list, and we’re waiting for more evidence of a global effect on brain injury symptoms), at a recent BART Foundation board meeting, it was decided that we should feature TMS as a safe, FDA-approved treatment for stubborn, re-occurring depression.
Depression is widespread among TBI/ABI survivors (35% according to CDC), and it is the premier co-morbidity contributing to the suicide epidemic among veterans and non-veterans alike. For individuals experiencing prolonged or re-occurring depression, TMS may be a drug-free remedy.
What is TMS?
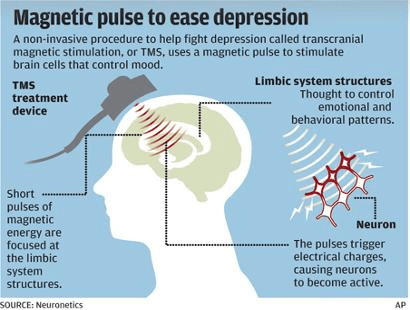
TMS is a non-invasive brain stimulation technique that uses magnetic fields to stimulate nerve cells in the brain. It is typically used when traditional treatments like antidepressants and therapy do not provide relief.
How It Works
- A coil is placed on the scalp, usually near the forehead.
- The device delivers magnetic pulses to the prefrontal cortex, a brain area involved in mood regulation.
- These pulses activate underactive brain cells, helping to restore normal brain function and improve symptoms of depression.
- TMS can be used alongside antidepressants or therapy.
TMS is performed while the patient is awake and does not require anesthesia. Each session lasts around 20-40 minutes, and a full course of treatment typically involves daily sessions for 4-6 weeks.
Current Research
TMS is a safe, effective, and non-invasive treatment that offers hope for people with treatment-resistant depression. While it is not a cure, many individuals experience significant improvements in their mood, energy, and overall well-being.
A search of the PubMed medical research database for depression and transcranial magnetic stimulation yields an incredible 440 clinical trials completed in the last year! While not all research involves TBI/ABI patients, there is hope for the future!
